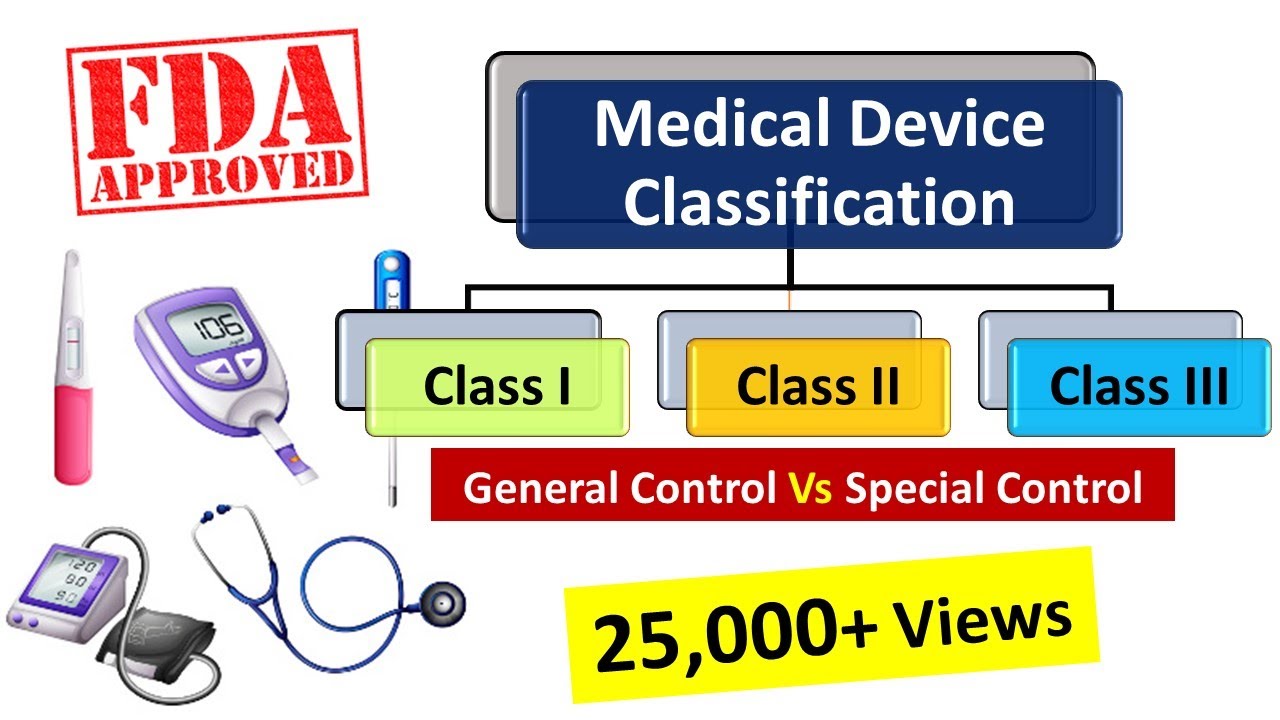
Medical Device Definition Us Fda . overview of regulations for medical devices: The information on this page is current as of mar 22, 2024. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. the food and drug administration (fda), an agency within the department of health and human services. definition of a medical device. Premarket notifications (510 (k)), establishment registration,. medical device amendments to the fd&c act was an intensive classification process that included. (continued) recognized in the official national formulary, or the united states.
from www.youtube.com
medical device amendments to the fd&c act was an intensive classification process that included. (continued) recognized in the official national formulary, or the united states. The information on this page is current as of mar 22, 2024. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. definition of a medical device. the food and drug administration (fda), an agency within the department of health and human services. Premarket notifications (510 (k)), establishment registration,. overview of regulations for medical devices:
Medical Devices classification as per FDA Medical Device Regulations
Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. the food and drug administration (fda), an agency within the department of health and human services. medical device amendments to the fd&c act was an intensive classification process that included. Premarket notifications (510 (k)), establishment registration,. definition of a medical device. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. overview of regulations for medical devices: The information on this page is current as of mar 22, 2024. (continued) recognized in the official national formulary, or the united states.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. (continued) recognized in the official national formulary, or the united states. medical device amendments to the fd&c act was an intensive classification process that included. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. The information on this page is current as of. Medical Device Definition Us Fda.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definition Us Fda overview of regulations for medical devices: medical device amendments to the fd&c act was an intensive classification process that included. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. The information on this page is current as of mar 22, 2024. the food and drug administration (fda),. Medical Device Definition Us Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. (continued) recognized in the official national formulary, or the united states. definition of a medical device. overview of regulations for medical devices: medical device amendments to the fd&c act was an intensive classification process that included. generic type of device means a grouping of devices that do not differ significantly. Medical Device Definition Us Fda.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. overview of regulations for medical devices: definition of a medical device. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. (continued) recognized in the official national formulary, or the united states. medical device amendments to the fd&c act was an intensive. Medical Device Definition Us Fda.
From www.slideserve.com
PPT Risk Assessments Patient Safety and Innovation PowerPoint Medical Device Definition Us Fda medical device amendments to the fd&c act was an intensive classification process that included. (continued) recognized in the official national formulary, or the united states. the food and drug administration (fda), an agency within the department of health and human services. definition of a medical device. Premarket notifications (510 (k)), establishment registration,. The information on this page. Medical Device Definition Us Fda.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Definition Us Fda overview of regulations for medical devices: Premarket notifications (510 (k)), establishment registration,. medical device amendments to the fd&c act was an intensive classification process that included. the food and drug administration (fda), an agency within the department of health and human services. definition of a medical device. generic type of device means a grouping of. Medical Device Definition Us Fda.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. medical device amendments to the fd&c act was an intensive classification process that included. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. the food and drug administration (fda), an agency within the department of health and human services. The information on. Medical Device Definition Us Fda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Definition Us Fda overview of regulations for medical devices: generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. definition of a medical device. (continued) recognized in the official national formulary, or the united states. The information on this page is current as of mar 22, 2024. medical device amendments to. Medical Device Definition Us Fda.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Device Definition Us Fda the food and drug administration (fda), an agency within the department of health and human services. (continued) recognized in the official national formulary, or the united states. The information on this page is current as of mar 22, 2024. definition of a medical device. generic type of device means a grouping of devices that do not differ. Medical Device Definition Us Fda.
From dxoobywxs.blob.core.windows.net
Diagnostic Medical Device Classification at Crouse blog Medical Device Definition Us Fda definition of a medical device. (continued) recognized in the official national formulary, or the united states. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. The information on this page is current as of mar 22, 2024. overview of regulations for medical devices: the food and drug. Medical Device Definition Us Fda.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Definition Us Fda the food and drug administration (fda), an agency within the department of health and human services. (continued) recognized in the official national formulary, or the united states. medical device amendments to the fd&c act was an intensive classification process that included. The information on this page is current as of mar 22, 2024. generic type of device. Medical Device Definition Us Fda.
From jamespaige.pages.dev
Top Medical Device Conferences 2025 James Paige Medical Device Definition Us Fda generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. overview of regulations for medical devices: definition of a medical device. medical device amendments to the fd&c act was an intensive classification process that included. The information on this page is current as of mar 22, 2024. Premarket. Medical Device Definition Us Fda.
From www.linkedin.com
FDA Regulatory Pathways for Medical Devices Medical Device Definition Us Fda Premarket notifications (510 (k)), establishment registration,. (continued) recognized in the official national formulary, or the united states. definition of a medical device. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. the food and drug administration (fda), an agency within the department of health and human services. . Medical Device Definition Us Fda.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Definition Us Fda (continued) recognized in the official national formulary, or the united states. The information on this page is current as of mar 22, 2024. Premarket notifications (510 (k)), establishment registration,. medical device amendments to the fd&c act was an intensive classification process that included. definition of a medical device. overview of regulations for medical devices: the food. Medical Device Definition Us Fda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definition Us Fda overview of regulations for medical devices: definition of a medical device. generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. (continued) recognized in the official national formulary, or the united states. medical device amendments to the fd&c act was an intensive classification process that included. Premarket notifications. Medical Device Definition Us Fda.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Definition Us Fda medical device amendments to the fd&c act was an intensive classification process that included. definition of a medical device. Premarket notifications (510 (k)), establishment registration,. overview of regulations for medical devices: the food and drug administration (fda), an agency within the department of health and human services. (continued) recognized in the official national formulary, or the. Medical Device Definition Us Fda.
From www.regdesk.co
FDA on Medical Device Reporting Specific Aspects RegDesk Medical Device Definition Us Fda generic type of device means a grouping of devices that do not differ significantly in purpose, design, materials,. overview of regulations for medical devices: The information on this page is current as of mar 22, 2024. medical device amendments to the fd&c act was an intensive classification process that included. Premarket notifications (510 (k)), establishment registration,. . Medical Device Definition Us Fda.
From www.arenasolutions.com
Medical Device Definition Arena Medical Device Definition Us Fda overview of regulations for medical devices: The information on this page is current as of mar 22, 2024. medical device amendments to the fd&c act was an intensive classification process that included. the food and drug administration (fda), an agency within the department of health and human services. generic type of device means a grouping of. Medical Device Definition Us Fda.